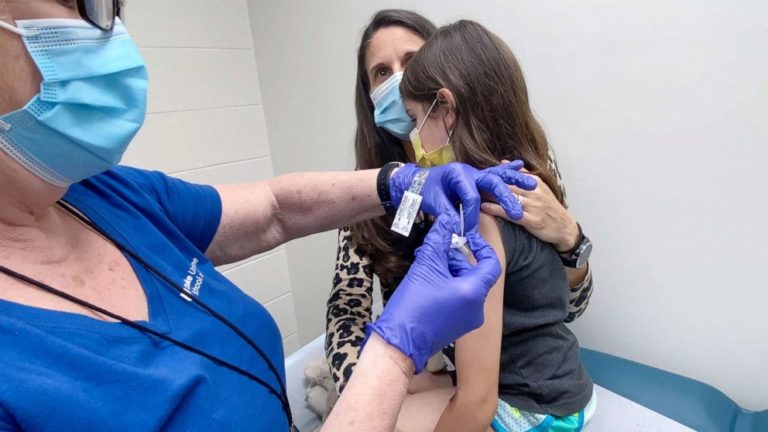
The US Food and Drug Administration (FDA) on Monday authorised the use of the Pfizer-BioNTech Covid-19 vaccine for children aged 12 to 15 years old.
Speaking yesterday President Joe Biden said; ‘This is a promising development in our fight against the virus’.
‘If you are a parent who wants to protect your child or a teenager who is interested in getting vaccinated, today’s decision is a step closer to that goal.’
The FDA previously granted an emergency use authorization for the Pfizer-BioNTech vaccine to individuals aged 16 and older.
Read Also: NAFDAC Approves Pfizer-BionTech Vaccine For Emergency Use
‘Having a vaccine authorized for a younger population is a critical step in continuing to lessen the immense public health burden caused by the Covid-19 pandemic,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
The FDA said some 1.5 million Covid-19 cases in individuals aged 11 to 17 years old have been reported to the US Centers for Disease Control and Prevention between March 1, 2020, and April 30, 2021.
The course of the disease is generally milder in children but they can pass it on to older, more vulnerable adults.
Pfizer and its partner BioNTech said in March that their two-dose vaccine regimen was shown to be safe and highly effective in a trial of 2,260 12 to 15-year-olds.
AFRICA DAILY NEWS, NEW YORK